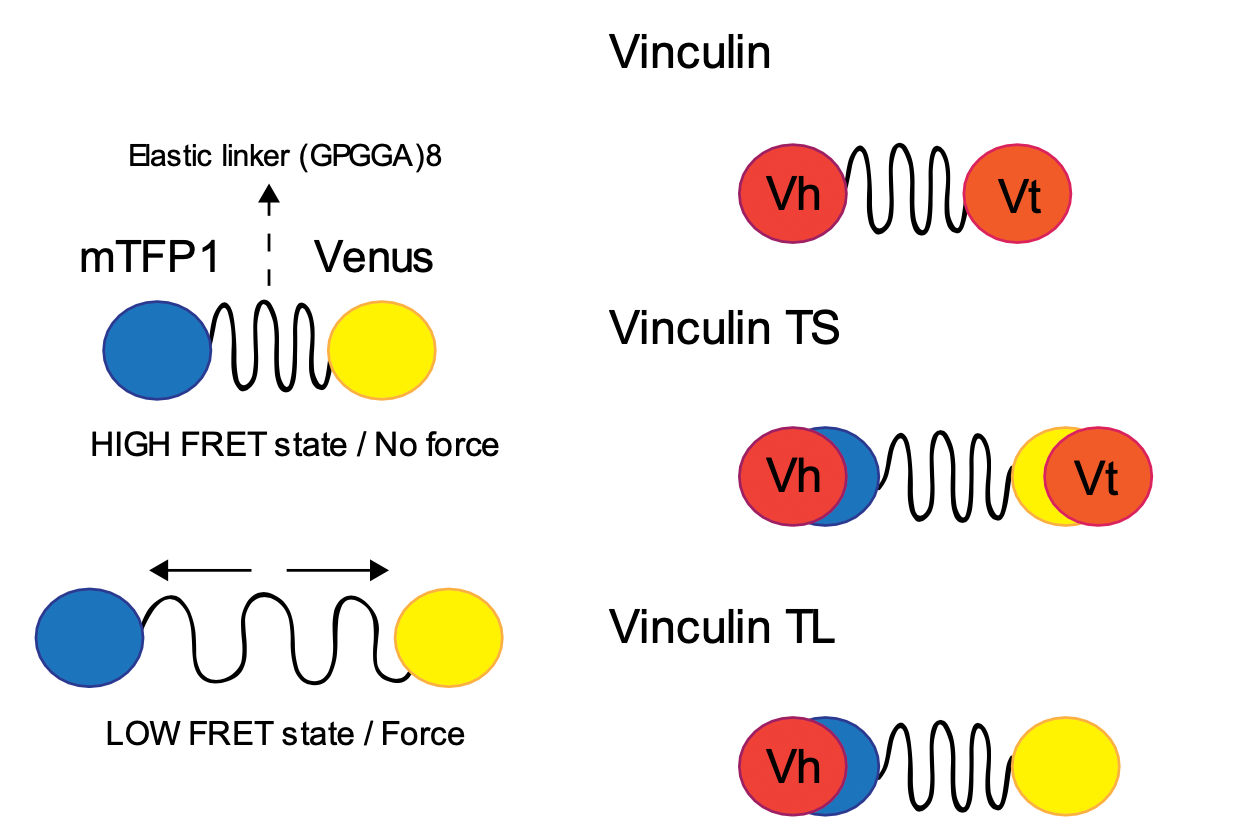
FRET-based force sensors
Mechanical forces play an undisputed elementary role in the interactions between cells and the surrounding extracellular matrix (ECM) [1]. Not only are these forces essential for the cells migratory behavior, they also influence proliferation (including tumor growth) and differentiation [2–4]. These forces are transferred across focal adhesions (FAs) which connect ECM and cell skeleton through patches of activated integrin proteins. Since the origin of exerted cellular forces lies in these FAs, they are the ideal starting point for characterization of mechanotransduction pathways. Consequently, studying how the properties of the ECM affect the cellular forces is key in understanding how cells connect to their environment and alter their behavior appropriately. While the scientific field of cellular mechanosensation has been studied for years, recent developments in imaging techniques and force sensor development enable us to dig deeper.